HCN Lewis Structure: A Simple Guide with Examples
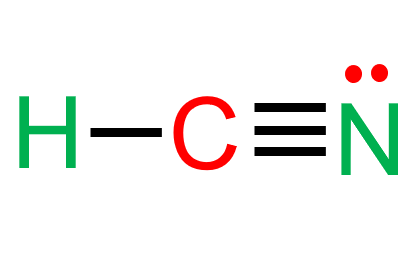

Hydrogen cyanide, or HCN, is a relatively straightforward molecule with several practical applications in the chemical, biological, and industrial sciences. The Lewis structure of HCN is presented here to illustrate the atomic arrangement and electron distribution within the molecule. Some of HCN’s features and applications will also be discussed.
Introduction
Hydrogen cyanide (HCN) is a linear molecule made up of three elements: hydrogen (H), carbon (C), and nitrogen (N). HCN has the chemical formula CHN. The centre element is carbon, which has a single bond with hydrogen and three triple bonds with nitrogen. There is one unpaired electron pair in the nitrogen atom.
HCN’s valence electron distribution is depicted in the molecule’s Lewis structure. The electrons in an atom’s outermost shell are called valence electrons. The number of valence electrons for typical elements is proportional to their position in the periodic table. Valence electron count: 1 for hydrogen, 4 for carbon, and 5 for nitrogen. One plus four plus five equals ten valence electrons in HCN.
The goal of this piece is to provide a step-by-step guide on drawing the Lewis structure of HCN. We will also go over some of the reasons why HCN is such a useful chemical in the fields of chemistry, biology, and industry. The dangers and toxicity of HCN to humans and animals, as well as its natural occurrences, will be discussed.
In this post, we will mostly discuss the following:
- What is HCN?
- Why is HCN important?
- How to draw the Lewis structure of HCN using six steps
- How to check if the Lewis structure of HCN is correct
You should be able to confidently sketch the Lewis structure of HCN by the end of this article, as well as appreciate its significance and relevance.
What is HCN?
Hydrogen cyanide (HCN) is a linear molecule made up of three elements: hydrogen (H), carbon (C), and nitrogen (N). The centre element is carbon, which has a single bond with hydrogen and three triple bonds with nitrogen. There is one unpaired electron pair in the nitrogen atom.
The following steps are required to depict the Lewis structure of HCN:
- Determine how many valence electrons are present in the molecule. The electrons in an atom’s outermost shell are called valence electrons. The number of valence electrons for typical elements is proportional to their position in the periodic table. Valence electron count: 1 for hydrogen, 4 for carbon, and 5 for nitrogen. One plus four plus five equals ten valence electrons in HCN.
- Single bonds should connect the central, least electronegative atom to the surrounding atoms. The electronegativity of an atom is a quantitative measure of its bonding attractiveness to electrons. A higher electronegativity indicates a greater tendency to attract electrons. Since among H, C, and N, carbon is the most electronegative, it is situated in the middle. For this reason, hydrogen is only attached to one side of the carbon atom. Carbon needs to be paired with nitrogen. At initially, we only employ single bonds to link them together.
- Take the total number of valence electrons and minus the amount of bonded electrons. Since two electrons are required to form a single bond, a total of four electrons have been consumed. There are a total of 10 minus 4 = 6 remaining electrons.
- Add extra electron pairs to the exterior atoms until each one has a full octet. A lone pair is a non-bonding pair of electrons. Around the atoms, they appear as a series of dots. Since the atoms in the periphery are more electronegative and prefer to have an extra octet, they get theirs filled first. To complete its octet, nitrogen has three additional pairs added to its side.
- Find out if the centre atom is missing an octet. If that’s the case, you’ll need to rearrange some lone pairs on the periphery to create more bonded atoms in the centre. To provide carbon the full complement of eight electrons, we add a third bonding electron pair by taking them from nitrogen.
- Verify that each atom has an exact number of electrons by counting them. If so, then our Lewis structure is right. Not unless we rearrange some electrons in our structure, of course.
HCN’s Lewis structure reveals the atomic arrangement and electron distribution of the molecule. It also aids in predicting the molecule’s polarity, acidity, and reactivity, among other qualities and behaviours.
Why is HCN important?
Having a bond angle of 180 degrees, HCN is a polar molecule. Since nitrogen and hydrogen have different electronegativity, the molecule is partially negatively charged on nitrogen and positively charged on hydrogen.
To a base, HCN can act as a weak acid by giving up a proton (H+). Its pKa value of 9.2 indicates that it is more acidic than ammonia (pKa = 36) but less acidic than water (pKa = 14).
Cyanides, nitriles, imines, and amides are just few of the many carbon-nitrogen-bonded organic molecules for which HCN is a precursor. This class of chemicals can be used in the synthesis of pharmaceuticals, polymers, dyes, and insecticides.
Some biological activities, such plant metabolism and bacterial fermentation, also produce HCN in nature. As a defence strategy against herbivores, several plants manufacture HCN. HCN can be used as a nitrogen source by creatures that can either detoxify it or tolerate it.
As a result of its inhibition of cytochrome c oxidase, an enzyme necessary for cellular respiration, HCN is also highly poisonous to most forms of life. Extremely high levels of HCN in the air can result in an instant death from suffocation. In some nations, HCN is also employed as a chemical weapon and as a means of execution.
HCN is a crucial chemical in many fields, including chemistry, biology, and technology. It’s a toxic chemical that can kill or harm animals and people. Therefore, knowing its make-up, characteristics, and applications is crucial.
Conclusion
Because of its versatility in chemistry, biology, and industry, we have spent this article learning how to sketch the Lewis structure of HCN. We are more aware of the risks associated with HCN and its potential applications.
Our intention was for this essay to be both educational and useful. Leave a comment below if you have any questions or thoughts. Your attention is appreciated.
You Can Also Read Here How Much is Big Ed Worth in 2023? A Detailed Breakdown of His Income and Expenses

 Top Five Factors for Choosing an Online Master’s Program
Top Five Factors for Choosing an Online Master’s Program  Top Five Reasons to Study in Australia
Top Five Reasons to Study in Australia  TTU Blackboard Tutorial: Essential Skills for Students and Faculty
TTU Blackboard Tutorial: Essential Skills for Students and Faculty  CCNA Dump: What It Is, How It Works and Why You Should Use It
CCNA Dump: What It Is, How It Works and Why You Should Use It  Suggested Answers for ICMAI: A Guide for Students and Teachers
Suggested Answers for ICMAI: A Guide for Students and Teachers  5 Ways a Free Resume Review Can Boost Your Career Prospects
5 Ways a Free Resume Review Can Boost Your Career Prospects  Stacey Dash Net Worth: Unveiling Her Financial Success
Stacey Dash Net Worth: Unveiling Her Financial Success  Stephon Marbury: A Basketball Journey from NYC to the NBA
Stephon Marbury: A Basketball Journey from NYC to the NBA  Best 5 AI-powered Voice Changer Tools for Creative Production
Best 5 AI-powered Voice Changer Tools for Creative Production  Rising Star: Frances Tiafoe Net Worth and Inspiring Journey to Tennis
Rising Star: Frances Tiafoe Net Worth and Inspiring Journey to Tennis